VAPE PMTA: FDA Registered Vape Brands Database
Estimated 0 min read
With industry changes that the PMTAs will bring, there is no doubt that every retailer involved in the vape industry should better prepare for the aftermath of the PMTAs.
Suppose you want to know which products have filed a PMTA or have been accepted, check our repository here. Or, if you’re a brand or manufacturer that has been PMTA pre-approved and would like to be listed, please apply here.
On September 9, 2020, the U.S. Food and Drug Administration’s (FDA) extended the deadline for vaping manufacturers to file their premarket tobacco applications (PMTAs) approached. These applications, which were the result of years of U.S. legislature, will give us a better understanding of which vape related products will legally remain on the market.
NOTE: If you’re already familiar with what the PMTA is and want to know which products have been accepted or pre-approved, you can visit the VapeRanger repository by clicking here.
Table of Contents
- What is the PMTA?
- What Does it Mean for the Vaping Industry and its Retailers?
- What Do You Need To Submit with a PMTA?
- Who Must Submit a PMTA with the FDA?
- What Products Should be Submitted?
- How Does the PMTA Review Process Work?
- Conclusion
What is the PMTA?
A PMTA is an application intended to provide scientific data that displays a product appropriate for public health protection. All new tobacco products that were not commercially marketed in the United States as of February 15, 2007, are required to have a PMTA filed to remain on the market.
The PMTA, in essence, will demonstrate that “a product is appropriate for the protection of public health” if it can show:
- The complete risks and benefits to the population the product creates
- Whether the product will help people stop using other tobacco products
- Whether people who currently do not use any tobacco products will be more or less likely to start using the product
- And finally, the way the product is manufactured, including controls, methods, and facilities used.
What does it mean for the vaping industry and its retailers?
There is no doubt that the PMTA will cause some changes to the vaping industry. While small players might fail to turn in PMTAs, we believe regulation of the sector could bring about safer and higher quality products that will, in the end, help better the reputation of vaping.
With a regulated market, vaping could shed the damaging misinformation surrounding it and transition to a healthier way of enjoying tobacco; one deemed more beneficial than smoking cigarettes.
As the FDA only approves the companies who have done their due diligence both in research and manufacturing, only vaping products that meet FDA standards will remain in the market, creating better vaping experiences for consumers in the United States.
VapeRanger has created an online repository of brands and products that have filed PMTAs. As PMTAs are pre-approved or accepted by the FDA, the repository will notate these critical updates. With this online repository, we hope to keep our clients up-to-date with the latest listings of products that are safe, legal, and in compliance with PMTA regulations.
You can visit the repository by clicking here.
What you need to submit with a PMTA
The PMTA application was thorough and strict, and it required many documents. From scientific studies to environmental assessments, the list of particulars was exceptionally long. For example, Juul’s application was 125,000 pages long. But in essence, all the information submitted needed to show the following:
- Full reports of all information published displaying the health risks associated with the product and whether it is less risky than other products on the market
- A complete list of components, ingredients, additives, and properties of the product
- A full description of methods, controls, and facilities used in the manufacturing processing, and the installation and packaging of the product
- Information that shows that the product meets the tobacco product standard, information that justifies any inconsistency from the norm, samples of the product, and labeling of the product
Who must submit a PMTA with the FDA?
According to the FDA, anyone that makes, modifies, mixes, manufactures, fabricates, assembles, processes, labels, repacks, relabels, or imports any “tobacco product” is considered a “tobacco product” manufacturer and therefore subject to submit a PMTA.
Even retailers that create their vaping products, including e-liquids, or components or parts, fall into the definition of “tobacco product” manufacturers, even if they don’t fabricate vapes. This is because the FDA’s definition of “tobacco products” is so broad.
While at first, “tobacco products” did not include e-cigarettes or its components, in May 2016, Congress created the “Deeming Rule,” which extended its definition of “tobacco products” to include electronic cigarettes as well as their components and parts.
With the Deeming Rule, almost all vaping related products fall into the definition of “tobacco products,” even e-liquids, which makes a vape shop, for example, that mixes and makes their e-liquid, a “tobacco product” manufacturer.
In the next section, we’ll go over all the vaping products considered “tobacco products” and therefore needed to be submitted to the FDA.
What products should be submitted to the FDA?
In the guidance document meant to assist persons or companies filing a PMTA as a vaping manufacturer, all of these products were required to be submitted for review:
- Additives: Some examples are flavoring or coloring common in e-liquids.
- Components or Parts: Some examples include atomizers, batteries, digital displays, tank systems, coils, and even software.
- E-Cigarettes: or vapes, vape pens, cigalikes, and open and closed systems, etc.
- E-liquids: Including liquid nicotine, liquids derived from tobacco, or liquids intended for human consumption in the tobacco category.
- Finished Tobacco Product: This refers to a product that includes all parts mentioned sealed in packaging.
- New Tobacco Product: Any tobacco product released after February 15, 2007
- Tobacco Product: “Any product made or derived from tobacco and intended for human consumption, including any component, part, or accessory of a tobacco product,” needed to be reviewed, in essence, all the items described above.
How does the PMTA review process work?
The process to submit an application and ultimately obtain approval is a lengthy affair consisting of six parts:
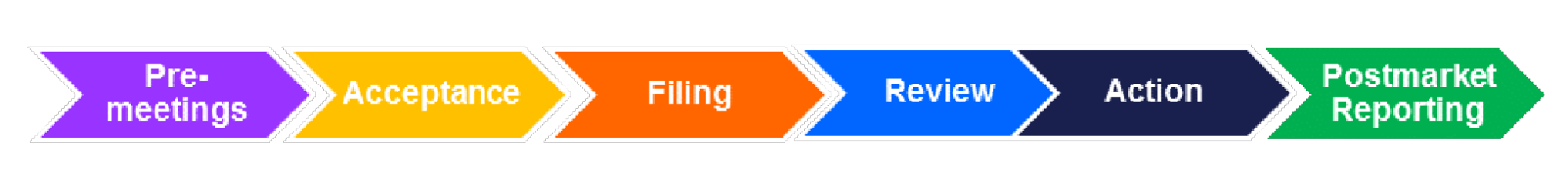
- First, a preliminary meeting between the manufacturer and the FDA must happen. During the Pre-meeting stage, the manufacturer met with the FDA and agreed on the best way to submit a PMTA.
- The second stage is the acceptance of the PMTA. In this stage, the FDA accepted or pre-approved the application, giving the company or manufacturer at least one more year to operate.
- In the third stage, the filing stage, the FDA will ensure that every item needed has been filed and the application is complete. In this stage, they might ask for additional information from the manufacturer.
- A substantive review will take place in the fourth stage. The FDA and the Tobacco Product Scientific Advisory Committee (TPSAC) will evaluate all the information provided to see if the product can be approved.
- In the fifth stage, the action stage, the manufacturer will either receive a marketing order to operate in the United States or receive a no-marketing order.
- Finally, in the last stage, the post-market reporting stage, the manufacturer will be responsible for maintaining reports and periodically contacting the FDA to ensure their product remains compliant.
Conclusion
We genuinely believe the new regulations will have a positive impact on the industry. What’s more, the PMTA process will make certain only legal, market-safe, and FDA approved tobacco products will make it to retailers and suppliers’ shelves.








